Table of Contents
Introduction: Why Medical Electronics Devices Are Booming in India
From wearable fitness trackers to IoT-enabled diagnostic devices, healthcare electronics are revolutionizing the way we monitor, diagnose, and treat health. In India, this transformation is gathering pace due to increasing demand for low-cost medical technology, government policies to spur local manufacturing, and the worldwide push toward telemedicine.
But here’s the truth: creating a medical device isn’t solely about conceptualizing an intelligent idea on paper. For Original Equipment Manufacturers (OEMs), the actual challenge starts when bringing that design into a safe, compliant, and reliable product. That’s where Electronics Manufacturing Services (EMS) partners come in.
What OEM Medical Devices Really Mean
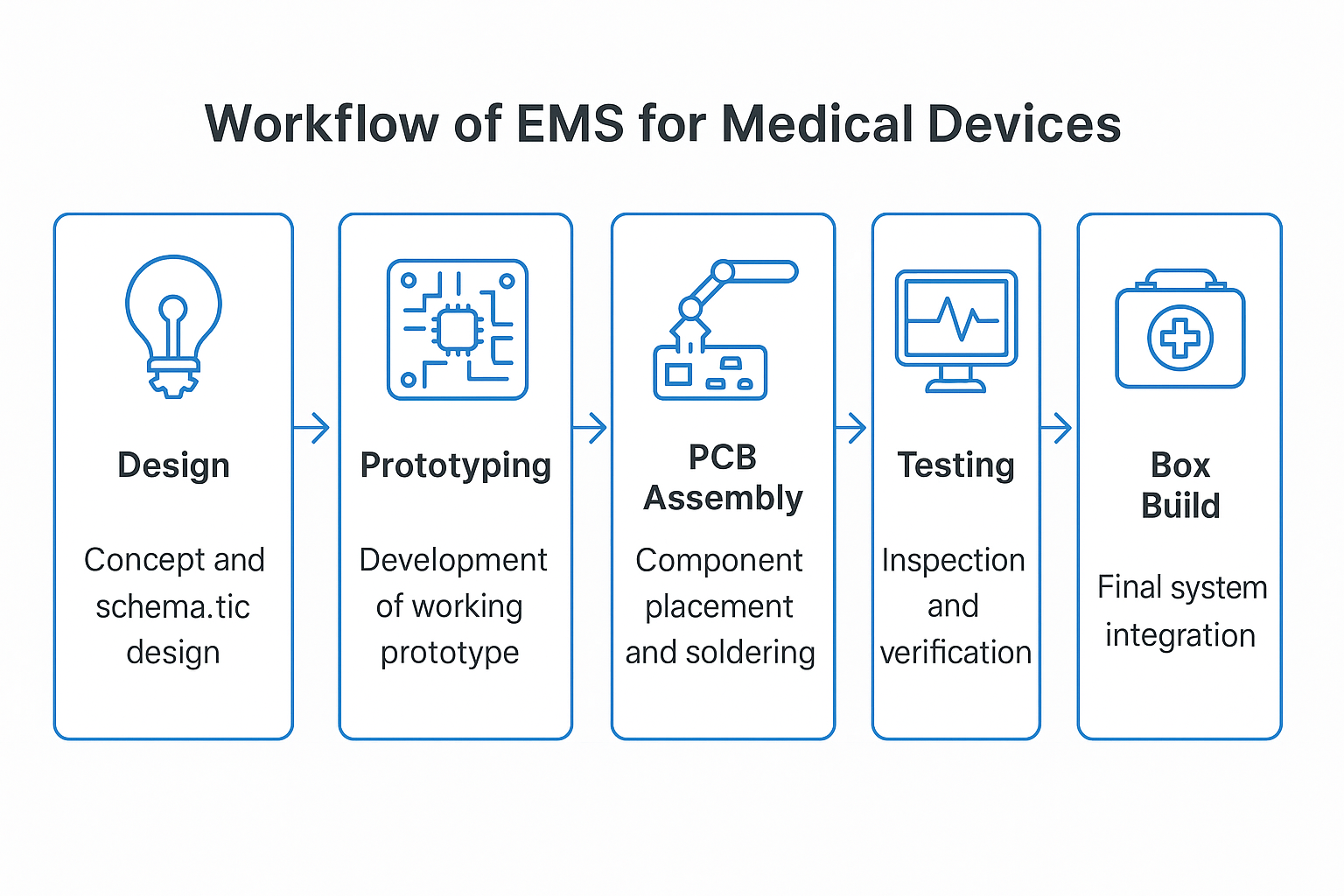
An OEM is really the designer of the product. They create the device, own the intellectual property, and determine how it should function. But rather than manufacturing every assembly or circuit board in-house, they tend to subcontract.
In the medical industry, OEMs depend on EMS providers for:
- PCB assembly (the device’s electronic brain)
- Prototyping (translating early designs into test hardware)
- Box build assembly (final assembly integration)
Consider it this way: the OEM gives the blueprint, and the EMS partner gives the shop qualified technicians and compliance experience to make it happen.
Challenges OEMs Encounter in Medical Device Production
Medical devices are not like consumer electronics. The quality and reliability standard is much higher because lives depend on it. OEMs encounter three large obstacles:
- Compliance & Standards
Regulations such as ISO 13485, IPC, CE, and UL are non-negotiable. Meeting them requires specialized processes and documentation. - Reliability & Zero Defects
A faulty circuit in a wearable tracker is frustrating. In a diagnostic device, it could compromise patient safety. That’s why stringent testing and traceability are critical. - Speed & Scalability
Startups and established OEMs alike must act fast – from prototype to market. Scaling up production, however, without sacrificing quality control, is challenging. - Supply Chain Concerns
Component shortages, extended lead times, and purchasing from trusted vendors add another level of difficulty.
How EMS Partners Overcome These Pain Points
This is where EMS providers such as MicroLOGIX are especially useful. Here’s how we bridge the gap between design and market-ready product:
- End-to-End Support: From design verification to box build, everything is done in-house.
- Quality Assurance: Each PCB undergoes Automated Optical Inspection (AOI), in-circuit testing, and functional tests before shipping.
- Compliance Expertise: Manufacturing according to ISO, IPC, and CE standards guarantees worldwide acceptance.
- Scalability: Either you require 10 prototypes or 10,000 units for production, EMS facilitates it without redefining the process.
The EMS Role in India’s Healthcare Innovation
India is fast emerging as a medical electronics device manufacturing hub on the global stage. Favorable engineering talent, cost-efficient operations, and government incentives through medical device parks offer an attractive option for OEMs.
The EMS partners are at the epicenter of this change. By taking on the complexity of production, they enable OEMs to concentrate on market strategy and innovation.
For instance, consider a startup building a next-generation wearable health monitor. The engineers are working on algorithms, sensors, and IoT Solutions for connectivity. The EMS partner is taking care of the PCB being assembled to precision, tested for compliance, and ready for scale. They both deliver a trustworthy product to the market – quicker and safer.
Why OEMs Select MicroLOGIX as Their EMS Partner
At MicroLOGIX, we’ve established our name on accuracy, dependability, and conformity. Here’s how OEMs rely on us with their medical and healthcare electronics:
- Design Validation to Manufacturing: We enable customers to easily transition from design validation to PCB Assembly and mass manufacturing.
- Advanced PCB Assembly: High-quality SMT and through-hole assembly supported by AOI and testing.
- IoT & Healthcare Solutions Expertise: Experience in creating electronics for connected healthcare devices through our IoT Solutions capability.
- Compliance-Ready Manufacturing: Processes that meet ISO and IPC standards.
- Local Presence, Global Standards: India-based, but with global quality expectations.
Conclusion: Building the Future of Healthcare Together
Medical electronics are building the future of healthcare, but it takes more than a clever idea to bring these devices into existence. OEMs require an EMS partner who can ensure quality, compliance, and scalability.
That’s where MicroLOGIX comes in. Together with us, you have a reliable manufacturing foundation that enables you to focus on innovation while we manage the complexity of production.
Frequently Asked Questions (FAQs)
-
What exactly does an EMS partner do in medical electronics?
Think of an EMS partner as the bridge between your design and a working device. They handle everything from PCB assembly and prototyping to compliance checks and final product build. In short, they make sure your idea turns into a safe, reliable, and ready-to-market medical device.
-
Why do OEMs usually outsource manufacturing instead of doing it in-house?
Setting up a medical-grade production facility is expensive and complicated. By working with an EMS provider, OEMs save time and money while tapping into proven expertise, quality processes, and certifications. This way, they can stay focused on design and innovation.
-
What certifications are required for medical devices in India?
Medical devices need to meet strict international standards like ISO 13485 for quality management, IPC standards for electronics reliability, CE for European markets, and UL safety certifications. Without these, your product can’t enter most regulated markets.
-
How do EMS companies make sure there are no defects?
Every board and assembly goes through multiple layers of testing – Automated Optical Inspection (AOI), in-circuit testing, and functional checks. The goal is simple: catch issues early, guarantee reliability, and deliver zero-defect products.
-
Why is ISO 13485 such a big deal in medical manufacturing?
Because lives depend on it. ISO 13485 lays down strict rules for how medical devices are designed and manufactured. If your EMS partner is certified, it means your product is built to the highest global standards for safety and reliability.
-
Can EMS providers in India handle both small and large production runs?
Yes. Whether you need just 10 prototypes or 10,000 finished devices, a good EMS partner can scale without cutting corners on quality. This flexibility is one reason India is becoming a go-to destination for medical manufacturing.
-
What’s the difference between OEM and EMS in simple terms?
The OEM designs the device and owns the idea. The EMS company builds it. You bring the blueprint, and they bring the tools, expertise, and compliance processes to make it real.
-
How do supply chain problems affect medical device production?
Component shortages or delays can slow down production, sometimes for months. EMS partners reduce this risk by sourcing parts from trusted vendors, planning, and keeping critical components in stock.
-
Why is India seen as a growing hub for medical electronics manufacturing?
It combines engineering talent, cost efficiency, and strong government support through initiatives like medical device parks. For OEMs, this means lower costs, faster turnaround, and products that meet global standards.
-
How does MicroLOGIX help with IoT-enabled healthcare devices?
We specialize in building electronics for smart healthcare – wearables, connected diagnostic devices, and remote monitoring tools. Our team ensures your device is compliant, reliable, and scalable, while you focus on innovation and user experience.

