Healthcare electronics are a different world compared to general consumer electronics. Devices used for monitoring, diagnostics, imaging, or therapy must perform with unwavering accuracy because real patient outcomes depend on them. That means the EMS partner you choose directly influences product reliability, regulatory approval, long-term lifecycle stability, and your overall time-to-market.
For OEMs and medical technology companies, selecting the right EMS provider is not just a procurement decision. It’s a strategic partnership that determines whether your device can be manufactured consistently, tested thoroughly, scaled efficiently, and validated for global medical markets.
This guide breaks down the essential factors healthcare OEMs should evaluate when choosing an EMS partner.
Table of Contents
Why the EMS Partner Matters More in Healthcare Electronics
Medical devices operate under higher stakes. A manufacturing error in a patient monitor, wearable health device, or diagnostic PCB has consequences far beyond product returns. Every build must meet strict regulatory expectations, pass rigorous testing, and maintain long-term reliability across changing environments.
A good EMS partner reduces your engineering burden, streamlines documentation, ensures safety compliance, stabilizes your supply chain, and supports you from prototype to full-scale production. A weak partner does the opposite: delays approvals, increases rework, and creates product risks.
Core Criteria Every Healthcare OEM Must Evaluate
1.Regulatory Compliance
Compliance isn’t optional in healthcare electronics. The EMS provider you choose must already operate within globally recognized medical-grade quality systems.
The most critical certification is ISO 13485, which defines the quality processes required for medical device manufacturing. Your EMS partner should also demonstrate:
- ISO 9001 for overall quality management
- IPC-A-610 Class 2 or Class 3 for PCB workmanship standards
- RoHS and REACH compliance for material safety
- CE or FCC readiness, depending on your market targets
Beyond certificates, what matters is whether the EMS maintains real documentation, discipline traceability logs, engineering change control, supplier validation, and risk management procedures.
A compliance-ready EMS partner reduces your regulatory load and ensures every build meets the expectations of notified bodies and certification agencies.
2.Manufacturing Quality and Testing Discipline
Medical-grade electronics demand precision and repeatability. Quality control can’t be something checked at the end; it must be built into every step of the process.
A capable EMS partner invests in:
- Automated Optical Inspection (AOI)
- X-ray inspection for hidden joints and BGAs
- Functional testing integrated with firmware
- Environmental Stress Screening (ESS)
- A documented CAPA (Corrective and Preventive Action) system
Industry experience consistently shows that many device failures originate in the design phase instead of assembly. A disciplined EMS ensures early prototyping, thorough testing coverage, and data-driven improvements that safeguard real-world performance.
3.Experience in Medical Device Manufacturing
Manufacturing for healthcare requires deeper expertise than standard electronics. The EMS partner should understand:
- Clean manufacturing workflows
- Low-noise analog PCB considerations
- Sensor integration and calibration routines
- Biocompatible and sterilization-friendly materials
- Regulatory documentation structures
Experience across categories such as patient monitoring, therapeutic systems, point-of-care devices, and medical wearables adds credibility. It also ensures the EMS already knows what clinical-grade reliability looks like.
4.Engineering Collaboration and Prototyping Support
Engineering collaboration is where an EMS can make or break your project. Medical electronics require early alignment on:
- DFM (Design for Manufacturability)
- DFA (Design for Assembly)
- DFT (Design for Testability)
- Component availability and lifecycle risks
- PCB-mechanical fit, enclosure constraints, and thermal behavior
Prototyping is not just a development step; it’s your first checkpoint toward certification-readiness. Strong EMS partners treat prototypes as engineering tools, uncovering issues that simulations miss EMI noise, sensor drift, connector tolerance mismatches, and more.
Teams that collaborate with their EMS early move through certification smoothly and with fewer redesign cycles.
5.Supply Chain Strength and Component Reliability
Medical devices often rely on long-life, stable components that are sensitive to supply chain disruptions. An EMS partner must demonstrate:
- Access to authorized distributors
- A clear counterfeit avoidance strategy
- EOL (End-of-Life) component monitoring
- Alternate sourcing plans
- Inventory and buffer planning for critical parts
A resilient supply chain keeps your production running even when global markets shift.
6.Scalability and Lifecycle Manufacturing
Medical electronics typically evolve through phases:
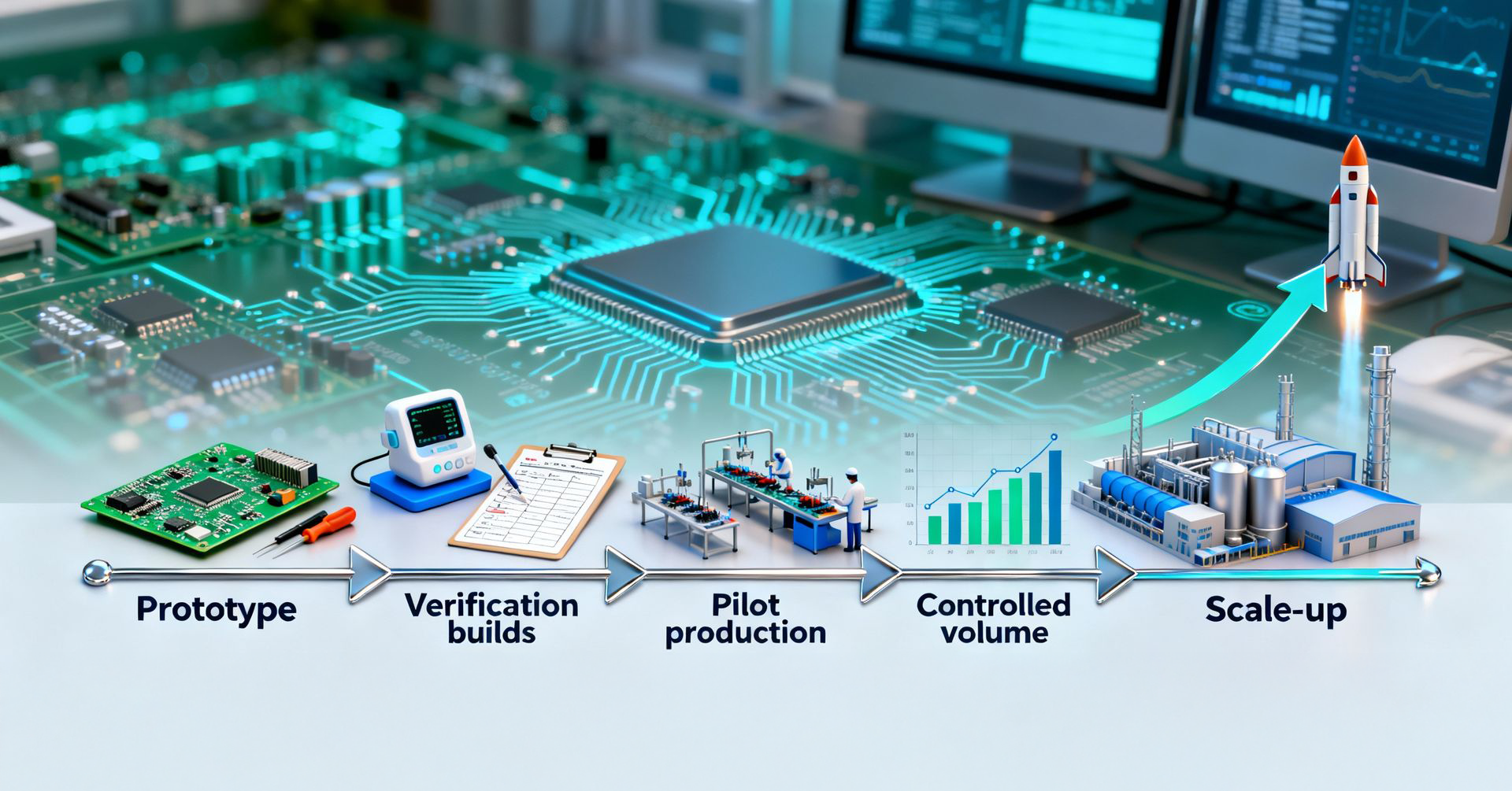 Your EMS provider must support all of these without compromising consistency. Look for:
Your EMS provider must support all of these without compromising consistency. Look for:
- High-yield SMT placement
- Multi-layer and high-density PCB capabilities
- Documented work instructions
- Traceability from component to final assembly
- Stable processes across small and large batches
Many scaling failures trace back to early design-stage decisions. EMS teams with strong engineering collaboration help you avoid those traps early.
Should You Choose a Turnkey EMS Partner?
Turnkey EMS solutions integrate sourcing, manufacturing, testing, documentation, and logistics under one ecosystem.
You benefit from:
- Unified quality and documentation
- Faster production cycles
- Fewer handoffs and communication gaps
- One accountable partner for all deliverables
For healthcare OEMs, this often means fewer certification delays and a smoother manufacturing journey.
Real-World Insight: What Today’s Healthcare OEMs Prefer
According to Emergo by UL’s Global Medical Device Manufacturing Survey, a majority of healthcare OEMs now prioritize ISO 13485-certified EMS partners as their first selection checkpoint. The same survey found that involving EMS engineers during early-stage design significantly shortens regulatory approval timelines due to better manufacturability alignment and fewer iterative redesigns.
Comparison Table: ISO 13485 vs ISO 9001
| Requirement | ISO 9001 (General Quality) | ISO 13485 (Medical Devices) |
| Focus | Customer satisfaction and quality management | Patient safety, traceability, and regulatory compliance |
| Design Controls | Recommended but flexible | Mandatory, documented, audit-ready |
| Risk Management | Not prescriptive | Must follow the ISO 14971 framework |
| Traceability | Basic | End-to-end device and component traceability |
| Regulatory Alignment | Indirect | Directly supports CE, MDR, and FDA reviews |
| Documentation | General QMS files | Technical files, DHRs, DMRs, CAPA discipline |
Conclusion
Selecting the right EMS partner shapes the entire lifecycle of your healthcare electronics device. By focusing on compliance discipline, manufacturing quality, engineering alignment, supply-chain reliability, and scalability, you position your product for stronger regulatory performance and consistent real-world results.
A strong EMS partner does more than assemble PCBs. They protect your timelines, strengthen your documentation, support your engineering, and reinforce the clinical reliability your end users expect.
FAQs
1. When should I involve an EMS provider in my medical device project?
Ideally, during the early design phase. Early EMS involvement improves manufacturability, reduces redesign cycles, and strengthens your ISO 13485 technical documentation.
2. ISO 13485 vs ISO 9001 – which one actually matters for medical devices?
ISO 9001 supports general quality practices, but ISO 13485 is essential because it aligns with medical regulatory expectations, documentation structures, and risk-management workflows.
3. How does turnkey EMS reduce FDA or CE marking timelines?
Turnkey EMS consolidates sourcing, manufacturing, testing, and documentation, eliminating handoff errors and producing audit-ready technical files faster.
4. What are the biggest red flags when evaluating a medical EMS partner?
Lack of ISO 13485 certification, poor documentation habits, unclear traceability, limited test capabilities, and little experience with IEC 60601-1, ISO 14971, or cybersecurity guidelines.
5. What does IEC 60601-1 mean for PCB design?
It defines essential performance and safety requirements for medical electrical equipment. Your EMS partner should understand how layout, spacing, insulation, and leakage current constraints influence compliance.
6. How does ISO 14971 integrate with EMS manufacturing?
ISO 14971 requires documented risk controls across the device lifecycle. A competent EMS partner integrates these controls into design reviews, testing workflows, and traceability systems.
7. How do EMS partners support FDA cybersecurity expectations?
They maintain secure firmware flashing practices, track software components, document update pathways, and align with FDA premarket cybersecurity guidance.
8. When is DFX most valuable in medical device development?
During prototype and verification planning. Strong DFX catches manufacturability issues before verification testing or regulatory submission.

